Unpacking the Data Sharing Dilemma in Clinical Trials: Are Researchers Really Sharing?
2025-09-01
Author: Benjamin
Introduction
A groundbreaking cross-sectional study sheds light on the often-overlooked aspect of data sharing in clinical trials. Conducted in line with the STROBE guidelines, this research delves into the discrepancies between data sharing plans registered during trials and those eventually published, raising crucial questions about transparency in clinical research.
How the Study Was Conducted
The researchers scoured the top ten medical journals renowned for publishing clinical trials, narrowing it down to six based on impact factors and relevance. These included prestigious names like The Lancet and the New England Journal of Medicine. Only trials initiated from January 1, 2019, onward were included to ensure compliance with International Committee of Medical Journal Editors (ICMJE) regulations, alongside publications appearing between January 1, 2021, and December 31, 2023.
Key Findings on Data Sharing Practices
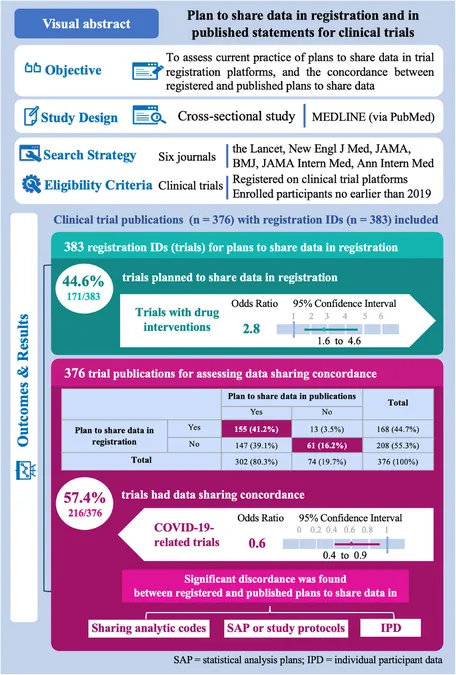
Of the 376 clinical trials analyzed, it was found that a staggering 44.6% had a plan to share data upon registration. However, this only highlights a part of the story: more than half of the trial publications lacked proper data sharing plans, indicating potential gaps in transparency. Alarmingly, over 40% of trials showed discordance in data sharing intentions between registration and publication.
Trial Characteristics and Their Impact
Trials involving drug interventions were significantly more likely to express a plan to share data. Conversely, COVID-19-related trials were associated with a decrease in data sharing concordance. Multicenter trials and those declaring conflicts of interest were more likely to maintain consistency between registered and published data sharing plans.
The Numbers Behind the Discovery
The study uncovered that less than half of the trials registered on ClinicalTrials.gov had comprehensive data sharing plans. Notably, while about 30% of trials reported plans to share individual participant data (IPD), there was a considerable drop in those willing to share statistical analysis plans and study protocols.
Call to Action: Improving Data Transparency
The findings underscore the pressing need for improved education on the importance of data sharing among researchers. Despite the ICMJE's recommendations aimed at promoting transparency, significant inconsistencies remain. The study advocates for a structured approach to data sharing in trial registrations and suggests improvements in how journals evaluate and report these plans.
Conclusion: Bridging the Gap
This analysis demonstrates that while awareness and intentions towards data sharing are growing, substantial discordance still exists between what is registered and what is published. Addressing these gaps is essential for the integrity of clinical research and the trust of the public.






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)