Unlocking the Secret to Immune Defense: How Calprotectin Starves Bacteria of Zinc
2025-09-22
Author: Jacob
A Battle Against Bacteria: The Role of Zinc
Zinc is essential for many bacterial enzymes, making it a prime target for our immune system. One of the simplest yet most effective strategies our body employs to combat infections is through calprotectin, a protein found in neutrophils that thrives at sites of infection. This remarkable protein has the ability to bind transition metals, effectively starving harmful microbes of zinc.
Revealing the Structure of Calprotectin and Zinc
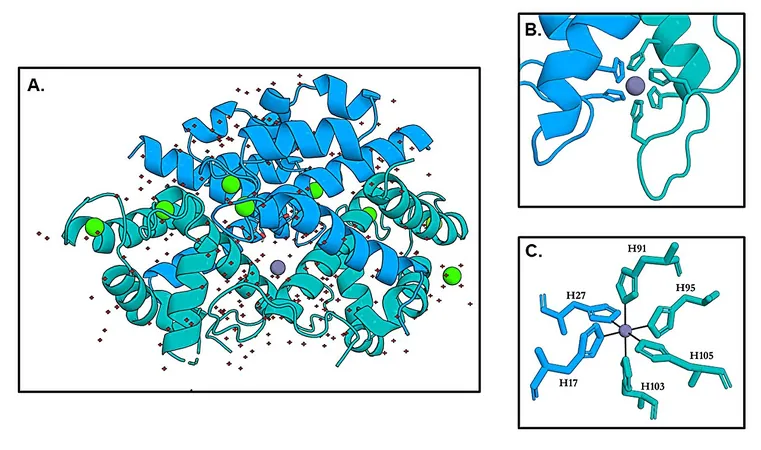
In an exciting breakthrough, researchers have unveiled the first crystal structures showing zinc bound to calprotectin. The zinc ion resides in a secure octahedral cage formed by six histidine residues from its two subunits, including a flexible C-terminal tail. At another critical site, zinc adopts a four-coordinate geometry, confirming how calprotectin secures this vital metal.
Unprecedented Affinity and Robustness
The researchers dove deeper to quantify how tightly calprotectin holds onto zinc. Astonishingly, even when the flexible tail—which enhances the binding—was altered or removed, calprotectin retained an exceptional affinity for zinc, holding it at picomolar levels. This highlights that even the structure's geometry plays a pivotal role in maintaining zinc's tight grip.
Impact on Pathogenic Bacteria
Focusing on the notorious pathogen Staphylococcus aureus, the study revealed that calprotectin significantly hinders its growth. When both metal-binding sites were disabled, this inhibitory effect diminished dramatically, underscoring the importance of metal sequestration in antimicrobial action.
Shaping Bacterial Communities and Growth Patterns
Beyond mere growth inhibition, the removal or alteration of the S100A9 tail changed the dynamics of how calprotectin interacts with bacterial communities, affecting biomass composition and adhesion patterns. This indicates that calprotectin does more than just withhold zinc—it actively shapes bacterial behavior.
Why This Discovery Matters
Understanding how calprotectin operates not only enhances our knowledge of innate immunity but also opens new avenues for medical intervention. Given that S. aureus biofilms can lead to severe complications in wound healing and device infections, insights into how calprotectin modulates bacterial growth could lead to novel therapeutic strategies.
Future Directions in Research and Treatment
Looking ahead, the findings suggest innovative design concepts for therapies. Histidine-rich structures that form upon binding could provide a blueprint for developing new zinc-immobilizing agents. Moreover, enhancing the interaction dynamics related to calprotectin may strengthen existing antibiotic treatments, steering bacterial cultures away from problematic biofilm formations.
Key Findings and Implications
The research highlights numerous significant advancements, including the first structures showing zinc bound to calprotectin, the confirmation that zinc is coordinated by all six histidines at the His6 site, and the persistent high affinity for zinc even without the tail. These insights deepen our molecular understanding of how the immune system curbs bacterial growth and refine our approach to combating stubborn pathogens.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)