Unlocking the Mysteries of Human Brain Cells: How Our Hippocampal Neurons Stand Out
2025-08-25
Author: Charlotte
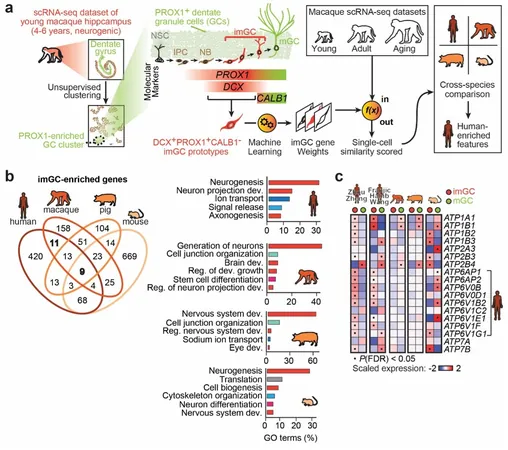
Neurogenesis—the process through which neurons are formed—has long captivated scientists, yet the intricate genetic and biological frameworks governing this phenomenon in humans remain largely uncharted. Recent advances at the University of Pennsylvania and the Chinese Academy of Sciences illuminate the unique characteristics of immature neurons, specifically immature dentate granule cells (imGCs), in the human brain.
Groundbreaking Study Reveals Unique Gene Patterns in Human Neurons
These imGCs are essential for brain adaptability, aiding in learning and memory throughout adulthood. In a study recently published in *Nature Neuroscience*, researchers examined how these cells develop and how their formation in humans diverges from other mammals such as mice, pigs, and monkeys. While the fundamental biological processes behind neuron development appear consistent across species, the gene expression profiles of human imGCs stand out significantly.
Yi Zhou, the lead author of the study, emphasized the critical role of these immature neurons: "imGCs arising from adult hippocampal neurogenesis in mammals are vital for brain plasticity and have immense potential in regenerative medicine." Yet, he lamented the existing knowledge gap on human imGCs, as most studies leaned heavily on mouse data, resulting in discrepancies when examining primates.
A Deep Dive into Molecular Features of Human Brain Cells
Zhou and his team devised a meticulous approach, analyzing single-cell RNA-sequencing data across four mammalian species—mice, pigs, monkeys, and humans—to unearth potential unique gene expressions specific to humans. They refrained from using mouse-derived markers to ensure precise identification of imGCs from human and macaque datasets.
Employing cutting-edge machine learning techniques, the researchers crafted an algorithm to accurately classify these neurons based on their molecular traits. By using young macaques as a benchmark, where imGCs are plentiful, they developed a classifier that successfully identified the transcriptomic characteristics of macaque imGCs.
Key Findings: Distinct Gene Expressions and Their Implications
The research revealed striking differences in gene expression richness in imGCs compared to mature granule cells across all species studied, although the biological processes underlying cell development remained consistent. Zhou pointed out that human imGCs showed enrichment in several gene families, particularly a notable ATP6 gene family involved in crucial cellular functions.
Their innovative human pluripotent stem cell model demonstrated how vacuolar-type ATPase plays an essential role in regulating the outgrowth and activity of imGCs, shedding light on their unique functionality.
Transforming Our Understanding of Neurogenesis Across Species
The findings from this pivotal research not only underscore the need for independent molecular analyses across species but also highlight the importance of human cell-based models. Zhou stated that such models could offer deeper insights into the regulatory traits of human imGCs, potentially informing future therapeutic strategies aimed at enhancing brain health.
Zhou hinted at the future direction of this research, suggesting that subsequent studies might focus on other critical brain cell types, moving beyond just imGCs to include quiescent and active adult neural stem cells. As the field progresses, larger sample sizes and multi-omics approaches could lead to groundbreaking discoveries, further unraveling the complexities of human neurogenesis.
In a world where understanding the nuances of our brain can lead to transformative medical advancements, this study is a beacon of progress, opening doors to a deeper grasp of our cognitive architecture.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)