Unlocking Nature's Power: Scientists Discover How Green Algae Repair Photosystem II
2025-07-08
Author: William
The Key to Life on Earth: Photosystem II Explained
Photosystem II (PSII) is a remarkable biological structure renowned for its ability to split water molecules using sunlight, producing oxygen in the process. This fundamental mechanism underpins global oxygen production and plays a pivotal role in converting solar energy into usable forms. Yet, despite its importance, PSII is vulnerable to light-induced damage, especially under intense lighting conditions.
The Mystery of Repairing PSII
Repairing PSII is complex, requiring the disassembly and reassembly of its intricate components alongside the replacement of the essential D1 protein found in its reaction center. While previous research provided insights into the initial stages of this repair process, the details behind its reassembly had remained largely mysterious until now.
Breakthrough Discovery Unveils PSII Repair Mechanism
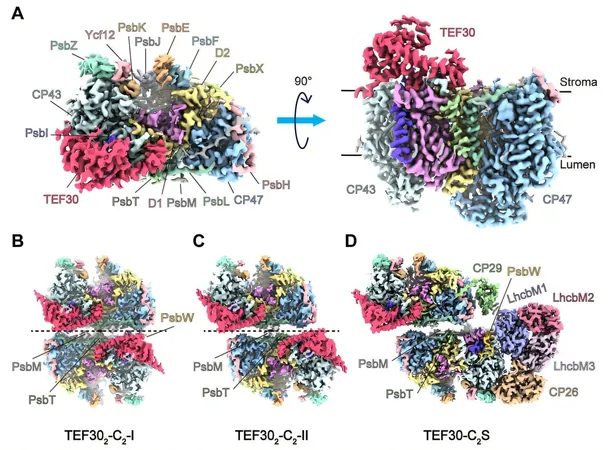
In an exciting breakthrough, a team of researchers led by Prof. Liu Zhenfeng from the Institute of Biophysics of the Chinese Academy of Sciences revealed the structural mechanisms that govern PSII repair in the model green alga Chlamydomonas reinhardtii. Published in Nature Plants on June 27, the study showcases four high-resolution cryo-electron microscopy (cryo-EM) structures that highlight the role of a crucial protein, Thylakoid Enriched Fraction 30 (TEF30). This protein is key in facilitating the repair and assembly of PSII.
Innovative Techniques Reveal Critical Insights
Employing a combination of innovative techniques like antibody affinity purification and sucrose density gradient ultracentrifugation, the team isolated PSII repair complexes associated with TEF30. Using single-particle cryo-EM, they uncovered detailed structures of four PSII intermediate complexes, named TEF30-C, TEF302-C2-I, TEF302-C2-II, and TEF30-C2S.
A Deeper Understanding of PSII Interactions
Structural analyses indicated that TEF30 attaches to the stromal side of the PSII core complex, forming strong interactions with four core PSII subunits: D1, D2, CP43, and PsbI. Biolayer interferometry assays further confirmed a high binding affinity between TEF30 and the PSII core.
Mapping the PSII Repair Cycle
By aligning and comparing the structures of the identified intermediate complexes with the mature PSII supercomplex, researchers identified several unique forms of PSII-C dimers for the first time. This comprehensive understanding is crucial, as it illustrates how various modules reassemble during the intermediary stages of PSII repair, eventually leading to a functional mature PSII supercomplex.
Implications for Photosynthesis and Agriculture
Prof. Liu emphasized the importance of these findings: "Understanding the roles of TEF30 in the PSII repair process is critical for advancing our knowledge of molecular events in photosynthetic organisms enduring constant light stress." He added that these insights not only provide a framework for further research on PSII maintenance but could also inspire methods to enhance the photosynthetic efficiency of crops, potentially revolutionizing agricultural practices.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)