Unlocking a New Era in Chemistry: Innovative Synthesis Strategies for Bridgehead Alkenes Break Decades-Old Rules!
2024-11-07
Author: Jacob
Groundbreaking Development in Chemistry
In a groundbreaking development, researchers have unveiled two novel synthetic strategies that successfully navigate the constraints imposed by Bredt's rule, allowing the creation of complex bridgehead alkenes for the first time. These pivotal advancements not only enable the synthesis of previously elusive compounds but also signify a significant leap forward in the pursuit of new drug candidates.
Understanding Bredt's Rule
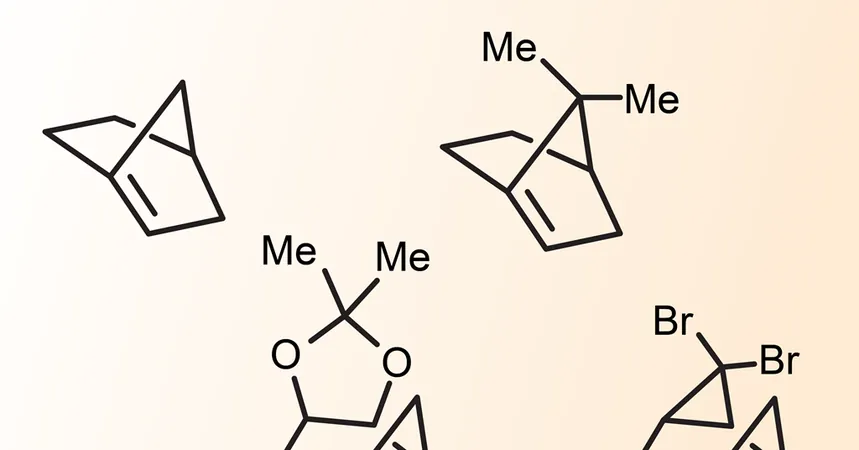
First proposed by chemist Julius Bredt over a century ago, Bredt's rule asserts that bridgehead alkenes are untenable due to structural issues in small-ring systems. The core challenge lies in the way a double bond at the juncture of two rings forces the molecule into a non-planar configuration, rendering it unstable and prone to undesirable side reactions.
Innovative Synthesis of Anti-Bredt Olefins
However, Garg's research team has taken bold steps to synthesize anti-Bredt olefins. By utilizing silyl-substituted precursor molecules—a method wherein a sizable trimethylsilyl group is attached to a carbon atom within a bicyclic architecture—this innovative approach allows for the effective generation of these complex structures.
By introducing triflate or trifluoromethanesulfonate, they create a powerful leaving group, which is subsequently released using tetrabutylammonium fluoride. Garg explains, 'You form a silicon–fluorine bond, which is very strong, and then you have a really hot leaving group,' making the synthesis of strained intermediates attainable under relatively mild conditions.
Trapping Complex Structures
In addition to synthesizing these anti-Bredt olefins, Garg’s team successfully trapped several complex structures by employing various trapping reagents, showcasing their newly established utility. 'Now, we’ve made ABOs [anti-Bredt olefins] synthetically useful,' Garg emphasizes, indicating that chemists can now integrate them into practical applications and consider them in synthetic design plans.
Chirality in Anti-Bredt Olefins
What sets anti-Bredt olefins apart from ordinary alkenes is their chiral nature, allowing these molecules to exist in non-superimposable mirror images. Garg and his colleagues managed to synthesize and isolate an enantiomerically enriched anti-Bredt olefin, confirming the transferability of chiral characters from precursor to product.
This advancement marks a pivotal moment in the realm of strained intermediates, as underscored by Courtney Roberts from the University of Minnesota, who is exploring the application of metal catalysts to harness functionalities in strained molecules.
Parallel Research on Hyperstable Alkenes
Meanwhile, parallel research led by Crag Williams at the University of Queensland has introduced a one-pot method for synthesizing hyperstable alkenes—cage-like structures anticipated to exhibit minimal reactivity despite their intricate geometries. Using a technique that involves a series of ring expansions involving boron intermediates, Williams's group successfully synthesized multiple bicyclic hyperstable alkenes.
Interestingly, contrary to expectations, these hyperstable alkenes displayed a degree of reactivity, prompting a reevaluation of existing criteria that suggest hyperstable structures should remain inert. Some of the bridgehead alkene structures investigated were found to be amenable to oxidation reactions, challenging long-standing assumptions about the relationship between stability and reactivity.
Divergent Reactivity Profiles
As Garg notes, 'What is cool and complementary is that in our study, the bridgehead alkenes are highly reactive, but in Williams's study, their bridgehead alkenes are stabilised,' illustrating how variations in molecular structure lead to divergent reactivity profiles.
Future Implications in Drug Development
Both teams are now poised to expand their investigations into the realm of strained alkenes, with potential implications for advanced applications in drug development and beyond. As Williams aptly concludes, 'Cage bicyclic systems are indeed very important for drug discovery, and thus having additional methodologies to access a broader range of cage systems will assist discovery chemists in their future pursuit of this goal.'
Conclusion
These revolutionary approaches not only redefine the theoretical boundaries of organic chemistry but also unlock new pathways for drug discovery and development, ushering in exciting possibilities in the field!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)