Unlocking a Green Future: Novel Method Transforms CO2 and Methane into Energy
2025-04-29
Author: Olivia
Revolutionizing Methane Conversion
In a groundbreaking development, researchers have forged a new path in the dry reforming of methane (DRM), a powerful process that converts carbon dioxide (CO2) and methane (CH4) into syngas. Traditionally, this process relies on a CO2/CH4 feed ratio of 1, but the increasing prevalence of CO2-rich natural gas calls for innovative solutions that bypass the costly separation processes typically required.
A Game-Changing Discovery
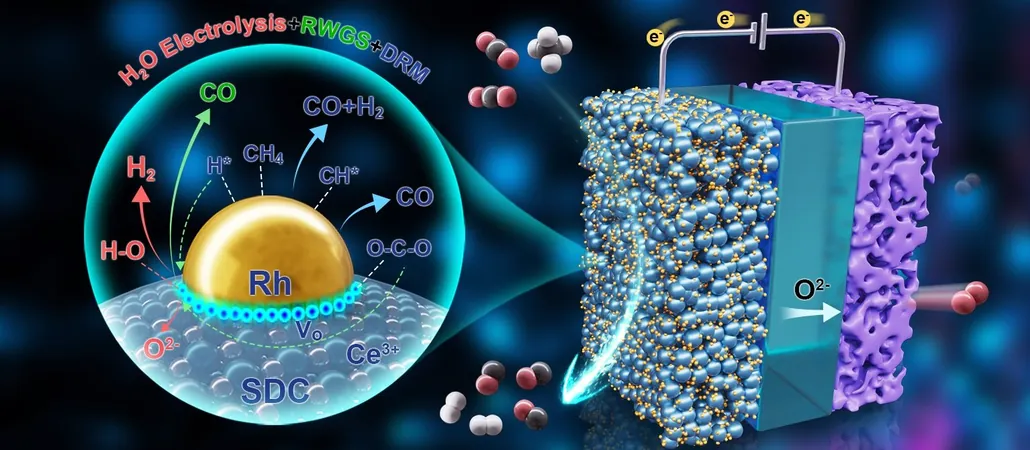
A team led by distinguished professors from the Dalian Institute of Chemical Physics has made significant strides in this area. Their recent study, published in the prestigious journal Nature Chemistry, unveils a new approach: super-dry reforming of methane, achieving a CO2/CH4 ratio of at least 2. This novel technique harnesses the capabilities of solid oxide electrolysis cells (SOECs) to directly convert CO2-rich natural gas into valuable syngas with remarkable efficiency.
The Power of SOECs
Operating at temperatures between 600 to 850 °C, SOECs are adept at transforming CO2 and water (H2O) into carbon monoxide (CO) and hydrogen (H2). This process boasts high reaction rates and energy efficiency, making it a beacon of hope for CO2 utilization, sustainable hydrogen production, and renewable energy storage.
Innovative Process Integration
By integrating traditional DRM with the reverse water-gas shift (RWGS) reaction along with H2O electrolysis directly at the cathode of SOECs, researchers have charted a new course. This method not only offers in-situ generation of H2 and O2 ions through the electrochemical reduction of water but also advances the RWGS equilibrium, significantly enhancing CO2 conversion and H2 output beyond what's typically achievable.
Outstanding Results
Testing the system at a CO2/CH4 ratio of 4, the researchers reported stunning results: a methane conversion rate of 94.5% and a CO2 conversion rate of 95.0%, all while maintaining nearly 100% selectivity for CO and H2. The apparent reducibility of methane reached a theoretical peak of 4.0, highlighting the method's efficiency.
Key Catalysts in Action
Further analysis confirmed that the Rh4+ sites were pivotal for methane dissociation. Meanwhile, the unique Ce3+-VO-Rh4+ interfaces, abundant in oxygen vacancies, played a critical role in facilitating CO2 adsorption and the RWGS reaction, while also driving the electrochemical reduction of water, boosting CO2 conversion and H2 selectivity.
The Future of Green Energy
This innovative approach not only stands to reduce greenhouse gas emissions but also paves the way for more sustainable energy solutions. With the potential to efficiently utilize natural gas that is rich in CO2, this research marks a significant leap towards a greener, more sustainable future.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)