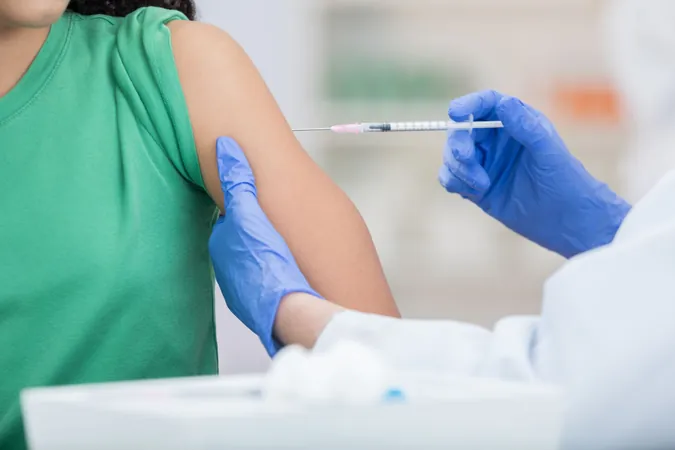
The Race Against Time: How Fall Vaccines Adapt to Evolving Viruses
2024-11-04
Author: Jacques
As the leaves turn and the autumn air settles in, the world gears up for vaccination season, with scientists tirelessly working to ensure the latest vaccines combat the ever-changing strains of influenza and SARS-CoV-2.
Every year, vaccines undergo significant modifications. This year, for instance, one particular flu strain that has been absent for several seasons has been excluded from some vaccine formulations. Moreover, the rapid evolution of SARS-CoV-2 necessitates continuous adjustments to the vaccines, demanding the attention of researchers globally.
Among those leading the charge are Dr. Manish Sadarangani and Dr. Julie Bettinger from the University of British Columbia's Faculty of Medicine. Dr. Sadarangani focuses on laboratory research and clinical trials of new vaccines, while Dr. Bettinger heads the CANVAS network, dedicated to monitoring vaccine safety post-approval.
The Process of Vaccine Development
When it comes to developing and optimizing vaccines each year, several key factors come into play. “Influenza and COVID-19 are driven by viruses that mutate over time,” explains Dr. Sadarangani. “With influenza, we see slight genetic variations annually, while for SARS-CoV-2, we are still understanding its pace of change.”
In the Northern Hemisphere, the selection of flu strains for vaccines takes place in March based on the strains that dominated the previous season and their anticipated evolution. Once the strains are identified, the vaccine production process spans about five to six months, resulting in vaccines ready for distribution by the fall.
The vaccine development process is rigorous and methodical. It begins in lab settings with extensive animal testing before progressing to human trials. Phase 1 involves a small group of healthy adults to assess safety, followed by Phase 2, where hundreds participate, including individuals from the target demographics. Finally, Phase 3 trials engage tens of thousands of participants, focusing on both safety and efficacy before the data is submitted to Health Canada for approval.
Canada’s Vigilant Approach to Vaccine Safety
Canada’s vaccine safety monitoring system stands out globally, especially with the implementation of the CANVAS network that collects electronic data directly from vaccinated and unvaccinated individuals. This contrasts with systems in Australia and the U.S., which often exclude unvaccinated groups, making it harder to identify potential safety signals.
Tackling Vaccine Safety Concerns
Dr. Bettinger points out that many safety concerns stem from misinformation and intentional disinformation. “Engaging with individuals through trusted healthcare providers in a safe and non-judgmental environment allows for meaningful conversations that can help dispel myths,” she notes, highlighting the importance of open dialogue in addressing public concerns.
The Future of Vaccination Strategies
As the scientific community grapples with the persistent challenges of viral evolution, researchers are investigating the feasibility of developing vaccines that could provide broader protection against multiple strains of flu and COVID-19. “This endeavor remains incredibly challenging,” Dr. Sadarangani acknowledges, “but we remain hopeful. In the next decade, we aim to make substantial advances that could lead to vaccines requiring less frequent updates than the current yearly schedule.”
In summary, as this vaccination season unfolds, it becomes increasingly clear that the journey of vaccine development is a crucial linchpin in our response to evolving viral threats. With dedicated experts and rigorous processes in place, there’s a continual drive toward a safer and healthier future. Stay informed and ensure you're ready for vaccination season!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)