Shocking Discovery: Graphite Instead of Diamond? New Research Turns Our Understanding Upside Down!
2025-07-09
Author: Jacob
Imagine if that sparkling diamond necklace was actually supposed to be made of the graphite found in your pencil. Sounds bizarre, right? But that's the intriguing reality researchers are uncovering!
New insights into how molten carbon transforms into either graphite or diamond are not just vital for geology; they hold implications for energy, material manufacturing, and even nuclear fusion! However, studying this crystallization moment has long been a scientific challenge due to the extreme conditions and rapid processes involved.
In a groundbreaking study published on July 9 in Nature Communications, experts from the University of California, Davis, and George Washington University have harnessed cutting-edge computer simulations to delve into how molten carbon crystallizes at temperatures and pressures akin to those found deep within our planet. Their revelations challenge established beliefs about diamond formation and illuminate the inconsistencies in previous experimental results.
Leveraging advanced machine learning techniques, the research team discovered that liquid carbon crystallizes in a far more intricate manner than we previously understood. In an eyebrow-raising twist, they found that graphite—commonly known as the soft form of carbon—can emerge spontaneously even when diamond is theoretically the stable outcome, possibly "hijacking" the diamond formation process!
Simulating the Depths of Earth’s Interior
The researchers created detailed models simulating conditions between 5 and 30 gigapascals (GPa) as molten carbon was cooled from a scorching 5,000 to a mere 3,500 Kelvin (K). As co-author and expert Donadio explained, such conditions might be replicated in laser heating experiments.
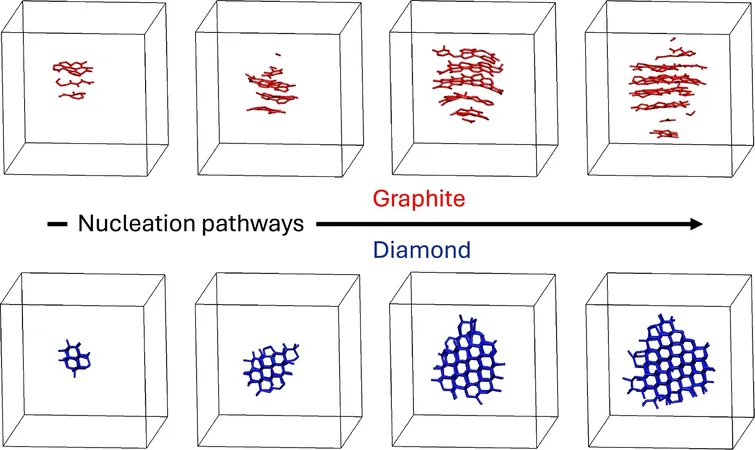
Rather than the expected glassy carbon from the rapid cooling, the team was astonished by spontaneous crystallization: at high pressures, carbon solidified into diamond, while at lower pressures, graphite took form. "This was a delightful surprise! Usually, simulating crystallization is more complex. We didn’t even have to employ tricks to achieve this," remarked Donadio. They were particularly struck by graphite forming at pressures up to 15 GPa, where diamond should typically reign.
Nature’s Path of Least Resistance
The researchers' findings comply with Ostwald's step rule, which states that crystallization can occasionally pass through intermediate phases rather than directly to the most stable form. In this case, graphite serves as a precursor to diamond, as its structural characteristics are more compatible with the liquid carbon's density and bonding.
As co-author Tianshu Li, a civil engineering professor, put it: "Liquid carbon finds it simpler to crystallize into graphite first, despite diamond being more stable in these conditions. Nature favors the easier path."
Unlocking the Mysteries of Crystallization
The simulations unveiled fascinating details about the molecular architectures of liquid carbon during its transformation. Graphite formed in elongated, column-like patterns, while diamond crystallized into compact structures.
This research provides answers to long-standing debates about high-pressure carbon experiments and paves the way for a clearer understanding of previously contradictory findings.
The implications are vast. Not only do they shed light on why natural diamond formation is so rare, but they also enhance our comprehension of the deep carbon cycle affecting Earth’s climate and geology over eons. Moreover, these crystallization pathways might revolutionize industrial diamond synthesis, particularly in high-precision fields like quantum computing.
"Understanding crystallization is crucial for technology, and diamonds serve as incredibly valuable materials," concluded Donadio. This work fundamentally changes our view on graphite's role in contexts where we least expect it!
The research was also supported by scientists Margaret L. Berrens, Wanyu Zhao, and Shunda Chen.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)