Revolutionizing Drug Analysis: A Breakthrough Method for Simultaneous Amlodipine and Aspirin Quantification
2025-09-23
Author: Emma
Developing a Game-Changing Analytical Method
In the quest for a reliable method to measure the concentrations of amlodipine and aspirin simultaneously, researchers have developed an innovative spectrofluorimetric approach. This solution not only addresses critical analytical challenges but also enhances the accuracy required for pharmaceutical quality control and therapeutic monitoring.
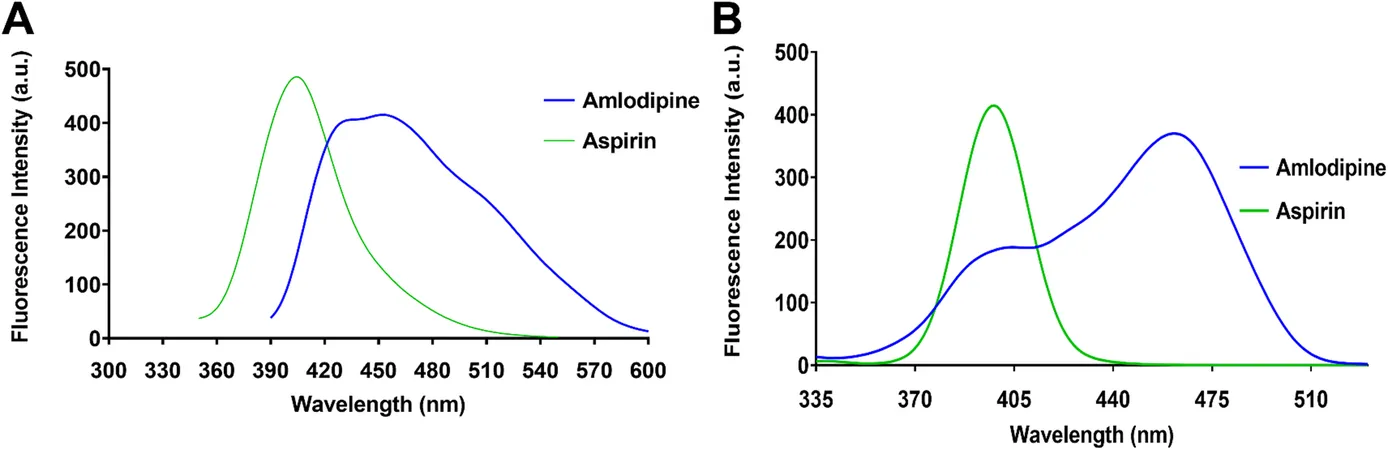
Confronting Spectral Overlap Challenges
A significant hurdle encountered in the analysis was the overlap in the fluorescence profiles of amlodipine and aspirin, complicating their direct quantification. When excited at optimal wavelengths, both drugs emitted overlapping fluorescence, posing a risk to the accuracy of traditional measurement techniques. Fortunately, researchers strategically employed synchronous fluorescence spectroscopy, adjusting Delta Lambda (Δλ) values to achieve superior spectral resolution.
Enhancing Signal with Smart Solutions
The team also investigated various solvents to boost fluorescence intensity, discovering that ethanol afforded the best results, allowing for significant enhancement of both drugs’ signals. They also optimized conditions using organized media like sodium dodecyl sulfate (SDS), marking a step forward in their analytical efforts.
Designing Robust Data - Calibration and Validation
To ensure that the new method would be both robust and reliable, meticulous calibration and validation datasets were constructed. The statistical soundness of the calibration process was confirmed, paving the way for a dependable calibration model employing partial least squares (PLS) regression.
Leveraging Intelligent Variable Selection
The breakthrough didn’t stop there. By implementing a genetic algorithm (GA) for optimal wavelength selection, researchers significantly reduced the complexity of their model while enhancing predictive performance. This innovative move allowed them to streamline data without compromising reliability.
Outstanding Validation Results
Validation against established methods revealed the superiority of the GA-PLS models over conventional approaches, showcasing phenomenal predictive accuracy and precision. This robust performance extends to analyzing real-world pharmaceutical formulations and biological samples, solidifying the method's practical utility.
A Step Towards Sustainability in Drug Analysis
The groundbreaking method also scored high in sustainability assessments, utilizing fewer toxic solvents compared to traditional methods, thus minimizing waste. The analytical performance, combined with its eco-friendliness, sets a new standard in pharmaceutical analysis.
Compelling Advantages Over Traditional Methods
When compared to existing methodologies for drug quantification, this new approach stands tall. It not only offers competitive detection limits and accuracy but does so in a way that prioritizes environmental impact and operational efficiency, marking it as a favorable alternative for routine pharmaceutical applications.
In Conclusion: A New Era for Analytical Chemistry
The introduction of this genetic algorithm-enhanced spectrofluorimetric method signifies a transformative leap in the analytical chemistry landscape. By facilitating simultaneous quantification of amlodipine and aspirin with remarkable precision and sustainability, it promises to redefine industry standards and improve patient safety in drug therapies.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)