Revolutionary Probe Breakthrough: Detecting Dangerous Hydrazine With Zero Background Noise!
2025-04-28
Author: Liam
Eliminating the Threat of Hydrazine
Hydrazine (N₂H₄) is not just another industrial chemical; it’s a highly toxic organic amine that can spontaneously ignite or even explode upon exposure to strong oxidants, air, or heat. Recognized as a Class B2 hazardous substance by the U.S. Environmental Protection Agency and other global health authorities, its inherent dangers have spurred the urgent need for effective detection methods.
The Challenge of Detection
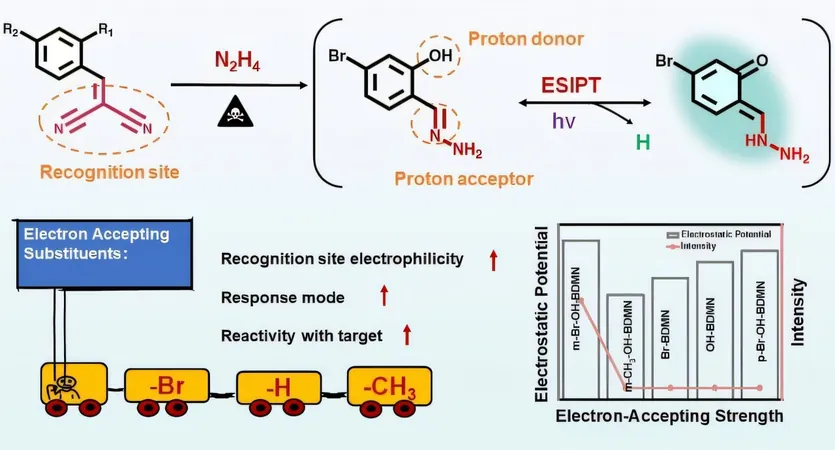
Existing fluorescence turn-on probes are designed using a sophisticated mechanism known as excited-state intramolecular proton transfer (ESIPT). While these probes boast remarkable sensitivity and reduced background noise, they often falter in specificity when faced with similar analytes. Additionally, there is a significant gap in research regarding how alterations in probe substituents can impact their efficiency in sensing hazardous substances.
A Game-Changing Solution from Cutting-Edge Research
Taking on the challenge, a dynamic research team led by Prof. Dou Xincun from the Xinjiang Technical Institute of Physics and Chemistry at the Chinese Academy of Sciences has unveiled an innovative zero-background fluorescence probe specifically engineered for the precise detection of hydrazine.
Engineering Detection: Probing the Future
Their groundbreaking study, recently published in the journal Analytical Chemistry, reveals a strategic approach to enhance the probe’s reactivity towards N₂H₄. By fine-tuning the electron-accepting ability of para-substituents and optimizing the positioning of proton donors, they have successfully boosted the sensitivity of the probe.
Introducing the Star of the Study: m-Br-OH-BDMN
The researchers crafted a series of zero-background fluorescent probes, including variants like m-Br-OH-BDMN, m-CH₃-OH-BDMN, and others, utilizing dicyanoethylene as the recognition site. A critical discovery was that the electron-accepting strength of the para-substituents, such as -Br, had a monumental impact on the probe’s effectiveness.
Unmatched Performance in Detection
Of all the probes, m-Br-OH-BDMN stood out, showcasing exceptional performance in detecting N₂H₄, delivering an astonishing limit of detection (LOD) of 0.46 nM (14.72 ng/L), a lightning-fast response time of just 1 second, and the ability to maintain superior selectivity even when confronted with 18 other similar substances. This breakthrough paves the way for safer industrial environments and more reliable detection methods, significantly reducing the hazardous risks associated with hydrazine.
Why This Matters for Safety and Industry
With such advancements, industries dealing with hydrazine can now adopt safer protocols, ensuring better protection for workers and the environment. This innovative probe not only highlights the power of science but also underscores the relentless pursuit of solutions to one of the most pressing challenges in chemical safety.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)