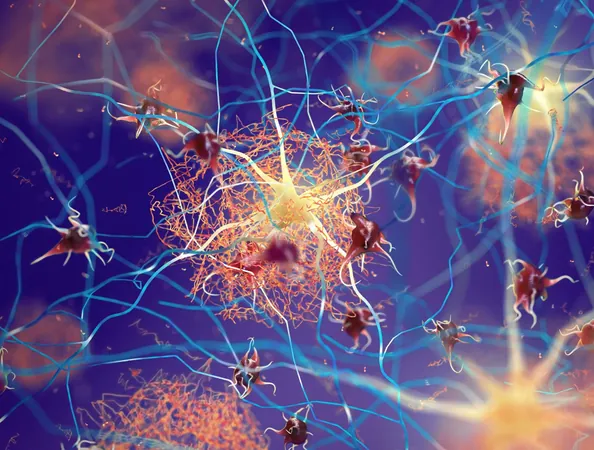
Revolutionary New Drug Shows Promise in Alzheimer's Prevention!
2025-03-21
Author: Liam
A Leap Forward in Alzheimer’s Research
The study was led by the distinguished Knight Family Dominantly Inherited Alzheimer Network-Trials Unit (DIAN-TU) at Washington University School of Medicine in St. Louis. The focus was on 73 participants with rare genetic mutations that drastically increase amyloid production in the brain, ensuring the onset of Alzheimer's disease by middle age.
For a specific subgroup of 22 participants who started the study symptom-free and received treatment for an average of eight years, the findings were staggering. The experimental drug was found to lower the risk of developing Alzheimer’s symptoms from a striking 100% to around 50%. This groundbreaking data hints at the potent potential of early intervention, though the exact duration of protection remains unknown.
Empowering Lives with More Healthy Years
Professor Randall J. Bateman, the study's senior author, expressed hope for these participants, emphasizing the importance of continued treatment. “While we don’t yet know how long these individuals can remain symptom-free, our goal is to provide them with the best chance of staying cognitively healthy,” Bateman stated. The prospect of delaying Alzheimer’s could not only enhance quality of life but also add valuable years to the lifespan of patients.
The Amyloid Hypothesis Gains Traction
These findings support the long-standing amyloid hypothesis, proposing that Alzheimer’s disease begins with the early buildup of brain amyloid plaques. By removing or preventing these plaques, researchers believe it's possible to stave off the dreaded onset of dementia. The study builds on the world's first Alzheimer’s prevention trial, initiated in 2012, which tested anti-amyloid therapies in participants showing no or very mild symptoms.
Despite prior findings indicating limited cognitive benefits from similar drugs, the recent trial has shifted the narrative. The data suggests that timely removal of amyloid could delay the onset of symptoms, particularly amongst those with no initial cognitive issues.
Exciting Future Trials on the Horizon
Following the study, participants were switched to lecanemab, a newly approved anti-amyloid treatment by the U.S. Food and Drug Administration, designed to slow cognitive decline in symptomatic Alzheimer’s patients. Furthermore, the new Knight Family DIAN-TU Amyloid Removal Trial is set to investigate lecanemab's effectiveness in preventing or postponing Alzheimer’s in families with high genetic risk.
While focused primarily on early-onset Alzheimer's, the research team hopes that insights gained will impact treatment across all forms of the disease, potentially preventing its onset in millions globally.
The Call for Optimism
Professor Bateman remains upbeat about the implications of these trials for the general population. “If the outcomes of late-onset Alzheimer’s prevention trials mirror those of our findings, we may soon have preventive options available for a broader audience.”
Although gantenerumab, a previously studied medication, has been withdrawn, other amyloid-targeting antibodies continue to be evaluated, fueling excitement for future breakthroughs in Alzheimer’s treatment.
As researchers uncover more promising therapies, the potential to change the trajectory of Alzheimer's for at-risk individuals across the globe grows brighter.
This significant study is detailed in the esteemed journal The Lancet Neurology, highlighting a pivotal moment in the fight against Alzheimer’s disease. The race is on, and the hope is real—could we soon see a world where Alzheimer’s is no longer an inevitability? Stay tuned for updates as this story unfolds!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)