Revolutionary Machine Learning Uncovers New Pathways for Hydrogen-Storing Superhydrides
2025-06-02
Author: Michael
Harnessing Hydrogen: The Future of Superhydrides
Superhydrides are emerging as game-changers in the realm of hydrogen storage, boasting the capacity to hold far more hydrogen than traditional hydrides. This makes them invaluable for high-tech applications, including hydrogen storage systems and superconducting materials for maglev trains and quantum computers. However, synthesizing these remarkable materials has proven to be a daunting task, requiring extreme pressures—on the order of tens of gigapascals—making the reactions challenging to manage.
AI Joins the Chemistry Lab: A Breakthrough in Synthesis
Now, a groundbreaking study has harnessed the power of machine learning to replicate the high-pressure synthesis of superhydrides. This innovation not only paves the way for finer control over superhydride production but also stands as a landmark example of machine learning’s potential to unlock unknown chemical pathways.
Published in the Proceedings of the National Academy of Sciences, this research showcases the extraordinary utility of AI in materials science. Professor Shin-ichi Orimo from the Advanced Institute for Materials Research (WPI-AIMR) remarked on the long, arduous journey to achieve the synthesis of calcium superhydride, sharing that the endeavor took a staggering decade to realize.
The Science Behind the Synthesis
As conventional synthesis techniques falter under high-pressure conditions, the lack of clarity surrounding these reaction processes has been a significant bottleneck. This study not only provides theoretical guidance essential for the advancement of superhydrides but also plays a crucial role in the quest for a carbon-neutral world.
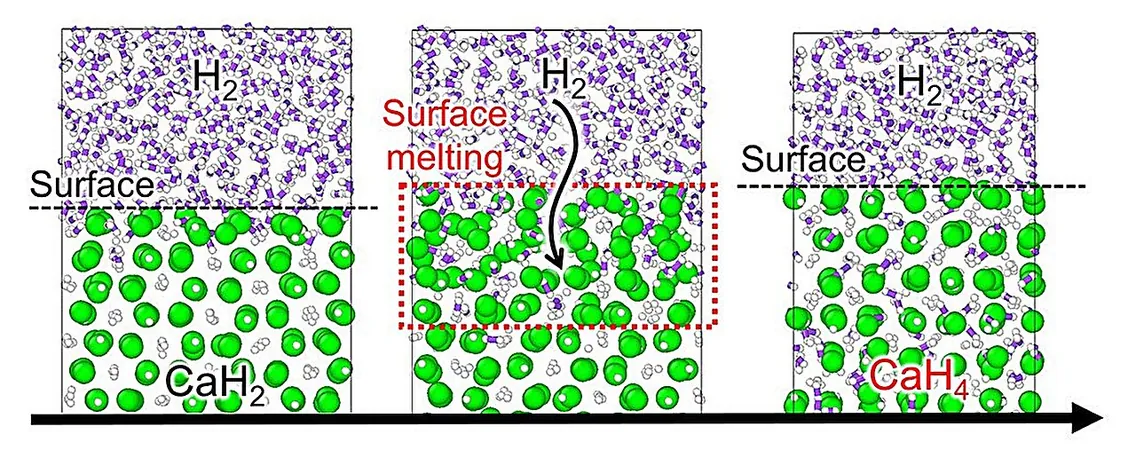
Under the leadership of Assistant Professor Ryuhei Sato from the University of Tokyo and in collaboration with other esteemed researchers, the team constructed a machine learning model trained on comprehensive data sets, including first-principle calculations of hydrogen and known calcium hydrides. This advanced model unveiled a novel reaction pathway where calcium hydride’s surface melts, absorbing hydrogen molecules at high temperature and pressure before crystallizing into bulk calcium superhydride.
Unlocking New Possibilities in Chemistry
The findings—illustrating surface melting due to pressure and molecular interaction, followed by hydrogen absorption—represent a significant discovery in high-pressure hydrogen chemistry. These insights broaden our understanding of critical physicochemical processes and emphasize the importance of easily calculable material properties, such as melting points, in determining reaction environments. This knowledge may streamline synthesis strategies, making superhydrides more accessible and feasible for development.
Professor Orimo encapsulated the importance of the work, stating that it establishes a new frontier for machine learning, capable of predicting previously unimagined chemical pathways and propelling materials science into a new era.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)