Revolutionary Editing Technique Transforms Rotaxanes by Removing Nitrogen Atoms!
2024-11-04
Author: Charlotte
Introduction
A groundbreaking new technique has emerged that selectively eliminates nitrogen atoms from rotaxanes, allowing for the formation of stable carbon–carbon bonds while preserving the integrity of the existing molecular structure. This innovative skeletal editing method not only pushes the limits of supramolecular chemistry but also opens the door to modifying numerous complex molecules that have been notoriously difficult to manipulate using traditional synthetic methods.
What are Rotaxanes?
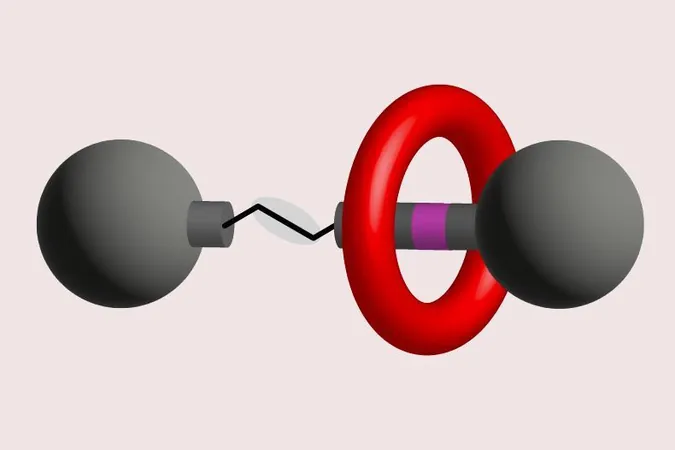
But what exactly are rotaxanes? These fascinating interlocked molecular structures consist of a ring-shaped macrocycle threaded onto a linear axle, which is secured with bulky stoppers preventing the ring from slipping off. This unique design enables rotaxanes to function as molecular machines capable of acting as switches and carriers for molecular cargo, making them highly valuable in various applications, including nanotechnology and drug delivery systems.
Research Team and Methodology
The research team, led by David Leigh from the University of Manchester, UK, has made significant strides by employing a skeletal editing technique to remove an amine site from the axle of a rotaxane. This specific amine site was crucial in holding the macrocycle in place during the molecule's construction. However, the challenge lies in that removing this template site is no small feat; the non-covalent interactions often keep the macrocyclic ring fixed around the axle, raising the risk of the ring slipping off and jeopardizing the entire structure.
Innovative Solution
To overcome this obstacle, Leigh and his colleagues built upon a pioneering method developed by Mark Levin's group at the University of Chicago in 2021. This method utilizes an anomeric amide reagent that facilitates the efficient removal of nitrogen from amine sites in larger molecules. Under alkaline reaction conditions, the macrocycle is temporarily shifted away from the amine site, granting the anomeric amide unobstructed access to the nitrogen atom.
Results and Confirmation
The success of this cutting-edge approach has been confirmed through a series of advanced techniques, including NMR, mass spectroscopy, and X-ray crystallography, which demonstrated that the unique interlocked shape of the rotaxane remained intact.
Implications of the Research
The implications of Leigh's research are significant. It not only enhances our understanding of molecular editing but also suggests that this technique could be employed to explore and modify a wide array of interlocked structures, such as catenanes and various types of rotaxanes featuring different motifs. This advancement might transform fields from materials science to biochemistry, heralding a new era in the manipulation of complex molecular architectures.
Conclusion
As researchers continue to push the boundaries of what's possible in supramolecular chemistry, we may soon uncover novel applications that can reshape technologies we rely on today. Keep an eye on this field — the future of molecular machines is just beginning to unfold!







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)