Revolutionary Discovery: Tiny MoOₓ Clusters Supercharge Methane Conversion on TiO₂ Nanosheets!
2025-07-02
Author: William
A Game-Changer in Photocatalytic Methane Oxidation
Scientists from the prestigious Innovation Academy for Precision Measurement Science and Technology in China have made a groundbreaking discovery: anchoring subnanometric MoOₓ clusters onto TiO₂ nanosheets can drastically enhance the selectivity for valuable organic products during methane oxidation while curbing CO₂ formation.
Tackling the Methane Challenge
Methane (CH₄) is abundant, yet its chemical inertness poses significant challenges for direct conversion into high-value products like methanol and formaldehyde. Traditional methods require harsh conditions and often lead to unwanted over-oxidation, making these processes inefficient.
The Green Promise of Photocatalysis
While photocatalysis powered by solar energy offers a more eco-friendly approach, achieving an ideal balance between activity and selectivity has remained elusive. Noble metals like gold may boost performance but come with a hefty price tag. This has led researchers to explore non-noble metal alternatives such as nickel and cobalt, yet striking the perfect balance between conversion rates and selectivity has been a struggle.
The Breakthrough Catalyst
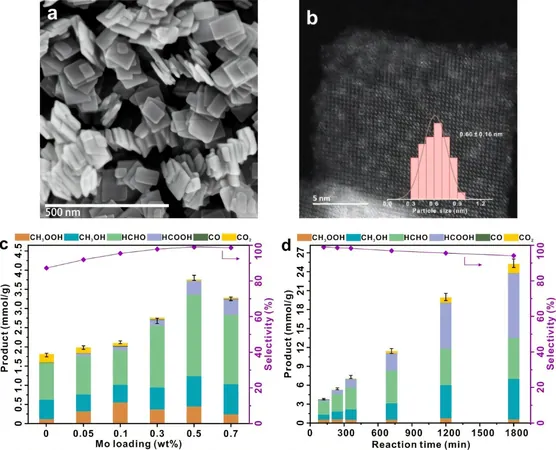
In a significant leap forward, researchers engineered a TiO₂-based photocatalyst adorned with ultra-small (0.6 nm) MoOₓ clusters. This innovative catalyst demonstrated remarkable efficacy in photocatalytic methane oxidation—specifically, with a 0.5% MoOₓ loading, it achieved a staggering organic oxygenate yield of 3.8 mmol/g in just two hours, boasting a selectivity rate nearing 100%! Additionally, it maintained an impressive apparent quantum yield of 13.3% at 365 nm and exhibited exceptional stability, retaining over 95% selectivity for over 1,800 minutes.
Unlocking the Mechanism: How It Works
Advanced techniques like in situ Electron Paramagnetic Resonance (EPR) and Nuclear Magnetic Resonance (NMR) revealed the secret behind this catalyst's performance: the MoOₓ clusters successfully activate O₂ to form surface-active species (Mo–OO and Mo–OOH). This activation facilitates the breakdown of methane's carbon-hydrogen bonds, forming key reaction intermediates (Mo-CH₂) and effectively preventing the formation of hydroxyl and superoxide radicals that cause over-oxidation.
Future Prospects
Furthermore, the photogenerated electrons play a crucial role in the reduction of Mo species, which, when combined with water, aid in the desorption of formaldehyde from the catalyst surface, further minimizing unwanted reactions. This research, published in Nature Communications, presents an exciting new frontier in the quest for efficient methane utilization, potentially paving the way for sustainable fuel production and environmental benefits.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)