Revolutionary Discovery: Fluorination Technique Unleashes Insight into Salinomycin's Conformation!
2025-05-28
Author: Benjamin
Serendipitous Breakthrough in Antibiotic Research
Researchers at Johns Hopkins University stumbled upon a groundbreaking method to selectively fluorinate the C–H bond in salinomycin, a potent antibiotic. Led by Thomas Lectka, the team’s unexpected findings allow for monitoring salinomycin’s conformation through 19F NMR technology, opening doors to new realms in biochemistry.
Salinomycin: A Dual Threat to Cancer and Antibiotic Resistance
Salinomycin is not just any antibiotic; it’s a large polycyclic molecule with promising anticancer properties. With an established role as an antibiotic, researchers are eager to tweak its structure by adding fluorine to enhance its efficacy and study its conformational changes. While previous attempts involved adding pre-fluorinated segments, directly attaching fluorine to salinomycin's core had never been accomplished—until now.
Daring Experiments Yield Unprecedented Results
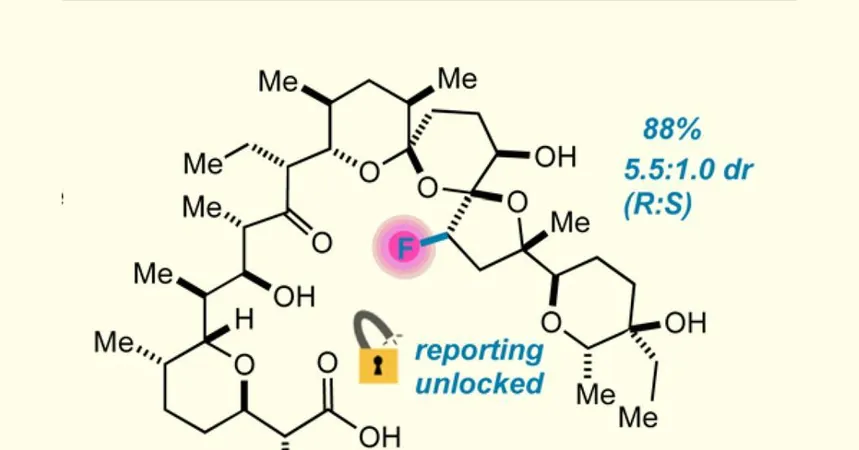
Lectka's team grappled with various methods to fluorinate salinomycin but faced challenges, including disintegration of its complex molecular structure. After numerous trials, the breakthrough emerged with a hydrogenated derivative, 18,19-dihydro salinomycin. This innovative approach allowed them to replace a C–H bond with a C–F bond, achieving remarkable regioselectivity, as Lectka explains: "Nothing else about the molecule changes."
The Science Behind the Success
The team discovered that the combination of Selectflour and a photoactive initiator generated a radical dication, facilitating a chain reaction that yielded 88% of their desired product. With a diastereomeric ratio favoring the R isomer, this discovery emphasized the crucial role of the carboxylic acid group in separating the molecule's conformers.
Conformational Dynamics Unlocked with Fluorine Tags
Graduate student Jonah Ruskin proposed using the fluorine tag to scrutinize salinomycin’s conformational dynamics. Ionophores like salinomycin tightly bind metal ions and sway into specific shapes, correlating with their biological efficacy. By employing 19F NMR, researchers could visualize interactions with alkali metals, finding that the fluorinated derivative favored potassium and sodium over lithium. Travis Dudding from Brock University further verified these findings through molecular mechanics calculations.
A New Era of Conformational Reporting
Ruskin asserts the uniqueness of this approach, stating that this fluorination technique is a pioneering step in conformational reporting—exploring dynamics with newfound clarity. Julian West, a synthetic chemist from Rice University, emphasizes the importance of understanding selectivity in C–H fluorination, highlighting the groundwork laid by this research for predictive advancements in the field.
Looking Ahead: Implications for Chemical Research
This research not only sets the stage for refining fluorination techniques but also aims to enhance our predictive capabilities in organic chemistry. The exploration of fluoro-tagging for conformational study expands the horizon of how complex natural product structures are understood, possibly transforming future drug development and chemical synthesis.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)