Revolutionary Breakthrough: New Technique for Detecting Hydrogen Isotopes in Titanium Hydride Nanofilms Could Transform Hydrogen Storage!
2024-11-14
Author: Olivia
Introduction
In an exciting development that could significantly impact hydrogen storage technologies, researchers from Japan have unveiled a groundbreaking technique for accurately locating hydrogen isotopes in titanium hydride nanofilms. As the smallest and lightest atom, hydrogen wields immense influence; its infiltration into various materials can alter their properties, including superconductivity and metal-insulator transitions.
Research Overview
The study, published in the prestigious journal *Nature Communications*, showcases the work of scientists from the Institute of Industrial Science at The University of Tokyo. Their research highlights an innovative approach for determining the positioning of hydrogen within nanofilms—a critical aspect for enhancing material efficiency in hydrogen storage applications.
Titanium Hydrides
Titanium is renowned for its ability to absorb hydrogen, forming titanium hydrides. Understanding the quantity and precise location of hydrogen atoms within these hydrides is vital for altering the material's characteristics to suit specific applications. Traditional methods like electron probes and X-ray techniques fall short in sensitivity, making the task arduous—until now.
Innovative Techniques
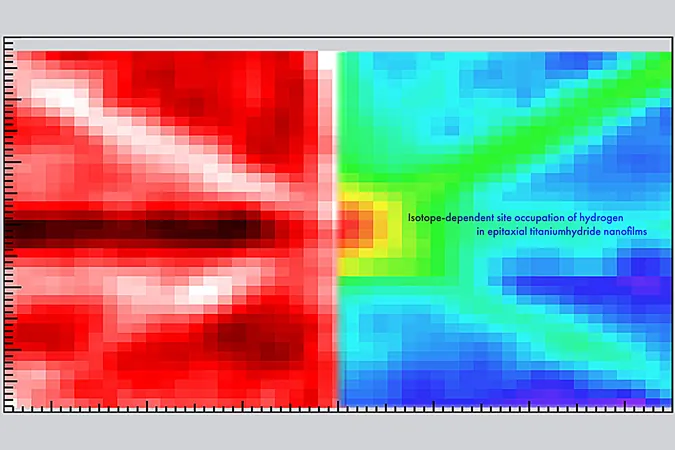
By integrating two advanced techniques—nuclear reaction analysis (NRA) and ion channeling—the researchers managed to create a detailed two-dimensional angular map of titanium hydride nanofilms. Lead author Takahiro Ozawa stated, “We focused on a TiH1.47 nanofilm because understanding such nanofilms is crucial, given that many hydrogen-related applications involve reactions at surfaces and subsurfaces. Our precision allowed us to accurately pinpoint both hydrogen and deuterium atoms in the nanofilm.”
Findings
The results revealed something remarkable: all deuterium (a heavier isotope of hydrogen) atoms occupied specific tetrahedral sites within the crystal structure of titanium. Surprisingly, 11% of the hydrogen atoms settled in octahedral sites. This diversity in atomic positioning resulted in reduced symmetry, hence increasing the stability of the lattice, a finding that could reshape material design in nanofilms.
Implications
What's particularly intriguing is that deuterium avoided octahedral sites due to nuclear quantum effects. This insight leads to the possibility of manipulating the ratios of hydrogen isotopes to fine-tune the stability and properties of nanofilms for targeted applications.
Conclusion
Katsuyuki Fukutani, the senior author of the study, emphasized the significance of this breakthrough: “Differentiating between the two isotopes in the hydride opens up opportunities for control. This will have profound implications for producing specific hydrogen-induced phenomena.”
Future Prospects
As the world seeks practical solutions for green energy, the researchers' enhanced understanding of titanium hydride nanofilms is poised to propel advancements in hydrogen storage technologies, solid electrolytes, and heterogeneous catalysis. This innovative method holds the promise of accelerating our journey toward safe and sustainable hydrogen solutions, making it a landmark achievement in materials science!
Stay Tuned
Stay tuned as this research unfolds—who knows what other revolutionary applications could be on the horizon?









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)