Revolutionary Biodegradable Nanoparticles Surge Ahead, Delivering Dual Drug Therapy Directly to Tumors!
2024-12-23
Author: Olivia
Introduction
In an exciting breakthrough, researchers from Tel Aviv University have unveiled a cutting-edge platform that utilizes biodegradable polymeric nanoparticles to deliver two drugs concurrently, specifically targeting various cancer types such as skin and breast cancer. This innovative method significantly boosts both the therapeutic efficacy and safety of treatments.
Research Findings
Published in Science Advances, this groundbreaking research led by Prof. Ronit Satchi-Fainaro addresses a critical challenge in cancer therapy: while combining drugs is essential for enhancing anti-cancer effects, discrepancies in their pharmaceutical properties—like degradation rates and ability to penetrate tumors—often hinder their simultaneous effectiveness.
Mechanism of Action
Prof. Satchi-Fainaro elaborates, "When multiple drugs are given at once, they typically don’t all reach the tumor site together, limiting their combined effects. Our investigation focused on ensuring that two drugs could be delivered together to the tumor while sparing healthy organs from damage."
Nanoparticle Design
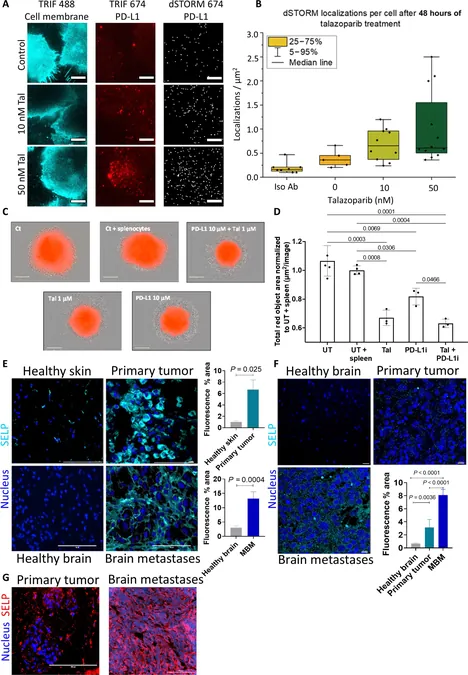
The team's innovative approach involves creating biodegradable nanoparticles that dissolve into harmless water and carbon dioxide within a month. These nanoparticles are ingeniously designed to encapsulate pairs of complementary drugs, working synergistically to boost each other's therapeutic activity. A key feature of this system is the attachment of sulfate groups to the nanoparticles, allowing them to selectively bind to P-selectin—an abundant protein found on cancer cells and newly formed blood vessels supplying tumors with essential nutrients.
Drug Combinations Tested
In their experiments, the researchers employed two FDA-approved drug combinations: BRAF and MEK inhibitors targeting melanoma (especially prevalent in patients with a significant BRAF gene mutation) and PARP and PD-L1 inhibitors aimed at breast cancers associated with BRCA gene mutations. The unique delivery system was rigorously tested in sophisticated 3D cancer cell models and in various animal models that mimic both primary tumors and their brain metastases.
Results and Efficacy
The results were remarkable. The nanoparticles demonstrated a high level of selectivity for targeting P-selectin, ensuring they accumulated predominantly in the tumor sites without affecting healthy tissues. Most impressively, they were capable of permeating the protective blood-brain barrier, effectively reaching and treating brain metastases while preserving the integrity of healthy brain tissue.
Comparison with Traditional Administration
Additionally, administering the drugs simultaneously via the nanoparticles proved to be significantly more effective than separate administrations, even at doses 30 times lower than those used in previous studies. Tumor sizes were dramatically reduced, and the treatment resulted in a staggering 2.5-fold increase in time to disease progression compared to standard therapeutic approaches, leading to a doubling of median survival rates in mice compared to those receiving standard drug treatments, and an impressive threefold increase when compared to untreated controls.
Conclusion and Future Prospects
Prof. Satchi-Fainaro emphasizes the versatility of this platform: "We successfully established an innovative method to deliver drug pairs to both primary tumors and metastases. Our findings indicate that this approach can substantially increase therapeutic efficacy in BRAF-mutated skin cancers and BRCA-mutated breast cancers, along with their respective brain metastases."
What's more, the design of the nanoparticle system permits it to be adaptable, capable of transporting various drug combinations aimed at enhancing the effects of treatments for a spectrum of tumors expressing P-selectin—including notoriously difficult cancers like glioblastoma, pancreatic ductal adenocarcinoma, and renal cell carcinoma.
This pivotal advancement not only represents a leap forward in cancer treatment but also opens doors to more effective therapies, potentially changing the landscape of cancer care as we know it. Stay tuned—this is just the beginning!






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)