Researchers 'Hack' Cancer Cells to Reveal Their Secrets: A Revolutionary Step in Immunotherapy!
2025-03-31
Author: Noah
In the world of technology, when a social media account starts to post bizarre or harmful content, it's a clear indicator that it has been compromised and needs serious intervention. Interestingly, a similar principle applies to our body's cellular mechanisms. Cells regularly communicate their status to the immune system by displaying a variety of proteins produced inside them. These proteins serve as a signal, allowing the immune system to identify and eliminate cells that display concerning behaviors, such as infection or cancerous transformation.
While healthy cells typically exhibit distinct proteins for immune recognition, cancer cells have a crafty survival tactic: they present very few unusual proteins, effectively masking their identity from immune detection. However, scientists from the Weizmann Institute of Science, led by Professor Yardena Samuels, have embarked on groundbreaking research that aims to change the game in cancer treatment by encouraging these cancer cells to 'show their true colors.'
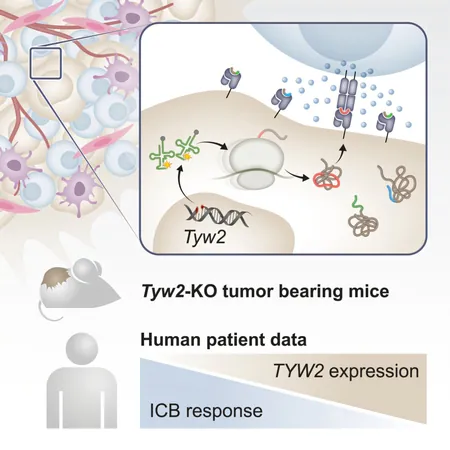
In a recent publication in Cancer Cell, the research team revealed an innovative strategy of artificially disrupting protein synthesis in cancer cells. This disruption causes these cells to produce a wide array of abnormal proteins, effectively alerting the immune system and triggering a robust immune response capable of targeting and killing human cancer cells. The results observed in mouse models were promising, demonstrating a significant slowdown in aggressive tumor growth.
The modern approach known as immunotherapy has transformed cancer treatment by harnessing the power of the immune system. However, a significant challenge remains: only a small percentage of patients benefit from these therapies, as many tumors lack the mutations necessary for identification by killer T cells—the immune soldiers tasked with eliminating malignancies.
Prof. Samuels shed light on an important aspect of their research: “The abnormal proteins aren't always the result of genetic mutations; they may arise from errors during the protein synthesis process itself.” In their study, the research team set out to intentionally disturb the translation process of proteins to expose more targets for immune system recognition.
This novel approach involved genetically engineering human melanoma cells to delete specific enzymes critical for accurate translation, leading to misunderstandings in the genetic code read by the ribosome—the cellular machinery responsible for protein production. This manipulation resulted in the creation of 34 unique short proteins, which could potentially serve as new immune targets.
The researchers then tested this methodology in mouse models, finding that disrupting translation in melanoma tumors triggered a stronger activation of killer T cells, significantly increasing their ability to infiltrate and attack tumor cells. Despite this, they faced a new hurdle: once these T cells reached the cancer, they were often exhausted and ineffective, a common drawback in immunotherapy.
To combat this, the researchers proposed a combination strategy. By pairing the new approach with existing immunotherapy treatments aimed at overcoming T cell exhaustion, they observed that an initially ineffective treatment drastically improved, leading to either full tumor eradication or significant shrinkage in approximately 40% of treated mice.
This pioneering study not only opens doors for potential new cancer therapies but also provides immediate insights for oncologists. Currently, immunotherapy is generally reserved for tumors with numerous mutations, leaving patients with fewer mutations in the dark. The researchers identified that low levels of specific translation-related enzymes could serve as a reliable predictor of immunotherapy success, offering hope to previously ineligible patients.
Looking forward, this research presents a fresh perspective on cancer treatment. Prof. Samuels noted, “Our findings show that disrupting the protein translation mechanism can amplify the immune response against tumor cells. With over 600 translation factors to explore, future treatments could target various aspects of the translation process across multiple cancer types.
Excitingly, ongoing collaborations with Stanford University involve utilizing AI technologies to discover additional potential targets within the cancer cell translation machinery. Plans are already underway to adapt this strategy to combat other cancers, including those of the breast, pancreas, and colon.
With fewer than 57% of cancer patients being eligible for current immunotherapy approaches, and only about 20% benefiting from treatment, this groundbreaking research has the potential to change the landscape of cancer care as we know it. Will this breakthrough signal a new era in the fight against cancer? Only time will tell, but with every discovery, hope for a solution grows stronger.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)