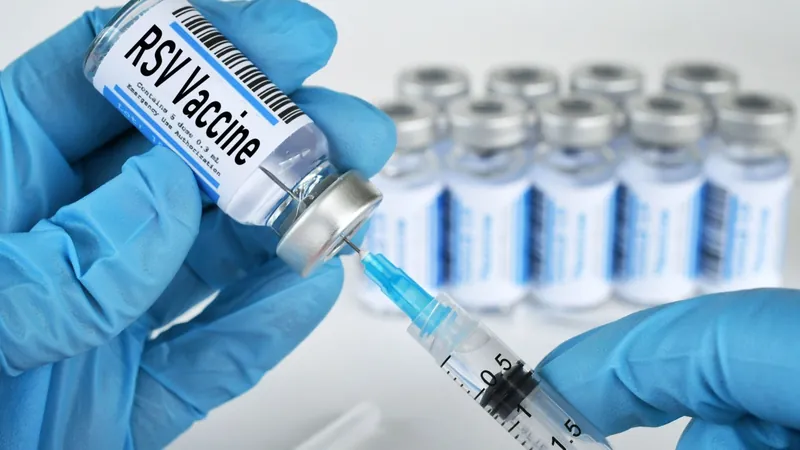
GSK's AREXVY Vaccine Gains Ground in Canada for Adults 50 to 59 – What You Need to Know!
2024-11-07
Author: Charlotte
Significant Approval for GSK’s AREXVY Vaccine
In a significant boost to public health, GlaxoSmithKline (GSK) has received official approval in Canada for its groundbreaking vaccine, AREXVY, aimed at preventing lower respiratory tract disease (LRTD) caused by the respiratory syncytial virus (RSV) specifically in adults aged 50 to 59 who are at an increased risk. This pivotal development is crucial as RSV, often thought of as primarily affecting infants, poses a serious threat to older adults and those with underlying health conditions.
Clinical Trial Insights
The approval springs from promising results obtained from a rigorous Phase III, randomized, placebo-controlled trial that monitored immune responses and safety across multiple countries. Notably, this study highlighted the potential of the RSV vaccine in bolstering the immune defenses of adults aged 50 to 59, a demographic that begins to show a natural decline in immune function, making them particularly susceptible to viral infections.
Expanding Reach
Not stopping there, GSK has also submitted regulatory applications to expand AREXVY's use in similar age groups in Japan and various other regions, with reviews currently underway by respective health authorities.
Further Trials
In an effort to widen its reach, GSK is conducting further trials to assess the efficacy of AREXVY in younger adults aged 18 to 49 who are at heightened risk for RSV-LRTD, as well as in immunocompromised individuals aged 18 and older. The results from these studies are anticipated to be unveiled by late 2024 and could pave the way for even broader vaccination recommendations.
Recommendations for Older Adults
Previously, AREXVY was already approved for use in adults aged 60 and older, and the National Advisory Committee on Immunization (NACI) has recommended its use for all adults aged 75 and above, along with residents in nursing homes and chronic care facilities aged 60 and above. Interestingly, adults aged 60 to 74 are encouraged to consult with healthcare providers regarding the benefits of vaccination against RSV.
Global Consensus on RSV Protection
GSK's expanded approval in Canada is in line with similar authorizations in the European Union and the United States, indicating a growing consensus on the importance of protecting older adults from RSV.
Importance of the Development
Michelle Horn, GSK's Interim Country Medical Director, emphasized the critical nature of this development, stating, “The natural age-related decline in immune function becomes more pronounced as we age, increasing vulnerability to viruses like RSV. Consequently, RSV-associated hospitalizations in adults start to rise at 50, complicating pre-existing health conditions.”
Conclusion on Vaccination Efforts
This approval marks a critical step towards enhancing healthcare for older adults, and with the ongoing trials and research efforts, the hope is to further mitigate the impact of RSV across a broader age range. Are you or someone you know within this age group? Don't wait—consult your healthcare provider today about getting vaccinated!






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)