Groundbreaking Discovery: Bacteria's New Anti-Viral Defense Mechanism Could Revolutionize Our Understanding of Immunity
2024-11-04
Author: Sophie
Groundbreaking Discovery: Bacteria's New Anti-Viral Defense Mechanism Could Revolutionize Our Understanding of Immunity
In a stunning revelation, scientists have uncovered a novel anti-viral defense mechanism in bacteria, fundamentally reshaping our understanding of microbial immunity. Just as complex multicellular organisms like humans fend off viral threats, single-celled bacteria are locked in an ongoing battle against their viral foes—bacteriophages, or phages. These viral entities, among the most prevalent life forms on Earth, incessantly attempt to infiltrate bacterial cells, prompting bacteria to develop sophisticated defenses.
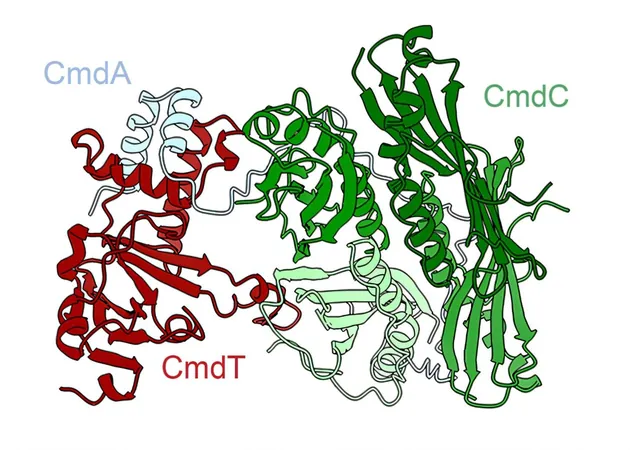
Recent open-access research from the Laub Lab at MIT, featured in *Nature*, introduces the CmdTAC system, an ingenious mechanism through which bacteria chemically modify their messenger RNA (mRNA) to thwart viral replication. This groundbreaking study not only reveals how bacteria recognize and respond to phage infections but also underscores the intricate evolutionary tactics they employ.
How CmdTAC Works: The Science Behind Self-Sacrifice
When under attack by a phage, the bacterial cell detects the presence of the viral invader at a critical point where the phage has hijacked the bacteria's cellular machinery. In a selfless act reminiscent of multicellular organisms, the compromised bacterium activates the CmdTAC system, effectively sacrificing itself to prevent the phage infection from spreading to its neighboring cells. “You could argue it’s a form of altruism where one bacterial cell puts itself at risk to protect others,” explains Christopher Vassallo, a research postdoc involved in the study.
At the heart of this defense mechanism is an enzyme known as an ADP-ribosyltransferase, which has now been characterized as capable of targeting mRNA—a feat never before documented in cellular biology. This study highlights a new layer of bacterial immunity that could have vast implications for both medical research and biotechnology.
The Role of Toxin-Antitoxin Systems
The CmdTAC system belongs to a broader classification known as toxin-antitoxin (TA) systems. These systems feature a lethal toxin that can halt or modify the cell's processes and an associated antitoxin that neutralizes this toxin under normal circumstances. The researchers identified CmdT as the toxic enzyme, which prompts the degradation of the antitoxin when a phage infection is detected, thereby allowing CmdT to restrict the production of new proteins essential for phage replication.
Christopher Doering, a co-author of the study, emphasizes the recent surge in understanding the complexities of anti-phage defense mechanisms, particularly through systems like CRISPR-Cas9, which has transformed fields ranging from genetics to medicine.
The Cutting-Edge Discovery of CmdT Activity
CmdT specifically recognizes and chemically modifies specific sequences of mRNA, particularly targeting sequences with the GA pattern. The modification effectively blocks protein translation, thereby stalling the infection at a crucial moment. “This study introduces a fresh perspective on bacterial immune responses, showcasing an entirely new endeavor—the ADP-ribosylation of mRNA,” remarks Vassallo.
Implications Beyond the Microscopic World
While the primary focus of this research lies within the realm of bacteria, there is potential for profound implications for human health. Senior author Michael Laub points out that homologous enzymes exist in eukaryotic organisms, including humans, where they may play roles in viral responses. Understanding these processes could lead to groundbreaking therapeutic approaches in combating viral infections and enhancing immune responses.
The exploration of bacterial anti-phage defenses not only broadens our understanding of microbial life but also tantalizes scientists with the prospect of uncovering shared mechanisms of immunity across diverse forms of life. As Laub succinctly puts it, “The thought that there may be commonality in how both bacteria and humans defend against viral threats opens up exciting avenues for research.”
In the face of an ever-evolving tapestry of pathogens, these insights could hold the keys to innovative treatments and preventive measures against viral infections—in both bacteria and humans—laying the groundwork for a future where we harness biological defenses to our advantage.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)