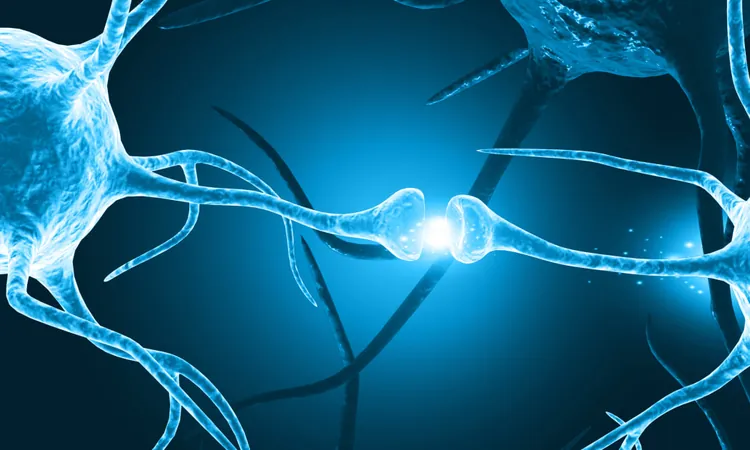
Breakthrough in Alzheimer’s Research: A Game-Changer Target to Combat the Disease?
2025-03-10
Author: Charlotte
Recent revelations from Yale School of Medicine (YSM) are setting the stage for a potential revolution in Alzheimer’s disease treatment, shifting the focus from conventional anti-amyloid therapies, which have largely yielded disappointing results. Researchers are now concentrating their efforts on a previously overlooked aspect of Alzheimer’s pathology: axonal spheroids—bubble-like formations that develop in neurons due to amyloid plaque accumulation.
In their groundbreaking study published on March 10 in Nature Aging, the YSM team, led by Dr. Jaime Grutzendler, alongside Dr. Harry Zimmerman and Dr. Yifei Cai, has pinpointed these axonal spheroids as critical players in the progression of Alzheimer’s. Unlike amyloid plaques, which have been the primary target of Alzheimer’s research, axonal spheroids contribute to the breakdown of neuronal communication by blocking electrical signals between neurons.
The study employed advanced techniques to dissect the complex molecular framework of these axonal spheroids, identifying potential avenues to reverse their detrimental growth. By collaborating with experts from the European Molecular Biology Laboratory, the team aimed to uncover the mechanisms that drive spheroid formation.
Grutzendler asserts, “Our research introduces a new hypothesis that axonal spheroids represent a significantly important pathological process. We believe that targeting these structures could open new therapeutic pathways that enhance neuronal function and restore brain circuitry rather than merely eliminating amyloid plaques.”
To achieve viable treatments, a deeper understanding of axonal spheroid formation is essential. The researchers posed fundamental questions, such as: “What causes a spheroid to form, and what are the pathways involved?”
Discovering the Mechanisms Behind Spheroid Formation
In an innovative approach, the researchers cataloged proteins within axonal spheroids, mapping out their interactions and the signaling pathways involved. By using antibodies that bind to specific proteins accumulating in the spheroids, they effectively created a network, unveiling hundreds of previously unidentified proteins that play a role in spheroid development.
One crucial pathway identified was the mTOR (mechanistic target of rapamycin) pathway, known for its engagement in cellular growth and metabolism. Experiments demonstrated that when this pathway was inhibited using pharmacological agents, the size of axonal spheroids notably reduced—a promising finding that could lead to meaningful therapeutic interventions.
A Future Filled with Hope for Alzheimer's and Beyond
The implications of this research extend beyond Alzheimer’s. Grutzendler notes that the presence of axonal spheroids could be relevant in other debilitating neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) and Parkinson's disease. The insights gained from this study could pave the way for novel treatment strategies not just for Alzheimer’s, but for a plethora of neurological conditions that share similar pathological features.
With plans for further exploration of the identified signaling pathways, Grutzendler expresses a keen interest in translating these findings into practical therapies that could transform patient care. “Our goal is to uncover specific therapeutic strategies to mitigate spheroid pathology and enhance neuronal function in the context of Alzheimer’s disease,” he concludes.
As this research unfolds, the scientific community and Alzheimer’s advocates are filled with renewed hope. Could this be the turning point in the long-standing battle against brain degeneration? Only time will reveal the answer, but the journey toward innovative therapies has begun.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)